|

Organic matter should be present on Mars from the infall of exogenous objects such as comets, chondritic meteorites, and interplanetary dust particles, and possibly also from indigenous biological or geochemical synthesis on early Mars. It has been estimated that long term infall has delivered up to ~ 104 g/m2yr of organic matter and probably includes; amino acids, nucleobases, alkylbenzenes, naphthalene and higher polycyclic aromatic hydrocarbons, carboxylic acids, and complex insoluble organic matter. Steininger et al. (2012) estimated that the Martian surface could contain up to 60-ppm organic carbon from meteoritic sources, although other estimates are as high as 0.2–2.9% (Flynn & McKay, 1990). PAHs may, however, oxidize (in the presence of ultraviolet radiation) to metastable benzenecarboxylic acids which may have accumulated up to 500 ppm in the top meter of the Martian regolith (Benner et al., 2000). If life was/is present, it would expand on abiotic molecular sources to produce more complex molecules, and more specifically, organic molecules which would provide chemical markers of past or present life. These molecules, or the the altered compounds or their fragments produced by diagenesis or interactions with oxidants and various ionizing radiation, are referred to as chemical biomarkers or just biomarkers [0]. A variety of such compounds or their fragments (e.g., hopanoids, lipids, chlorophyll, histidine, RNA/DNA) have been identified as being priority targets for life detection.
Given that exogenous infall, it was surprising when neither the Gas Chromatograph-Mass Spectrometer (GC-MS) on board the 1975 Viking Mars landers or the Thermal and Evolved-Gas Analyzer (TEGA) on the 2007 Phoenix Mars Lander detected any organic compounds in the martian surface samples [1,2]. However, when the Wet Chemistry Lab (WCL) onboard the Phoenix performed the first wet chemical analysis of the martian soil, in addition to determining a variety of soluble salts, the analyses revealed ~0.6 wt% perchlorate (ClO4-), most likely in the form of Ca/Mg(ClO4)2 [3-5]. The widespread occurrence of ClO3- and ClO4-, referred to as oxychlorines (ClOx), have subsequently been confirmed by their detection in the martian meteorites EETA79001 and Tissint [6,7] and by the Sample Analysis at Mars (SAM) instrument on the Curiosity rover [8]. The presence of the ClOx in the martian soil explains why no organic compounds could be detected by either the Viking GCMS or the Phoenix TEGA. Even if they had been present, they would have been combusted before reaching the mass spectrometer.
Under Mars ambient conditions ClO4- and ClO3- can be photochemically produced on Cl-bearing mineral surfaces, most likely due to silicate (SiO2) and/or metal-oxides acting as photocatalysts to generate radicals such as O2-, which can then react with chloride [9]. During this process (Figure 1) several ClOx intermediates such as hypochlorite (ClO-), chlorite (ClO2-), chlorate (ClO3-), and chlorine dioxide (ClO2) gas [9A], as well as radicals such as ClO-, O2-, ‧OCl, ‧Cl, and ‧OH are also likely generated [9-11]. These intermediate products can alter or fragment organic compounds, with only highly refractory and/or those well protected from UV surviving. Some of the intermediaries may be even more destructive than direct UV-driven reaction processes because they are not limited by regolith screening, and through diffusion, cryoturbation, or impact reworking, could over time reach to greater depths (e.g., chlorine dioxide gas [10] by diffusion). Understanding the formation of oxychlorines and the processes by which they or their intermediates alter or fragment organic compounds is key to understanding the preservation and detection of biomarker compounds. We have recently shown the fragmentation of tryptophan when exposed to UV in the presence of an oxychlorine [10A].

Figure 1. Reactive oxychlorines and radicals are produced, which
along with UV irradiation, can alter or destroy biomarker molecules.
|
In addition to the oxidation of organic compounds by oxychlorines, radiation in various forms is by itself a dominant factor. Even though much higher levels of solar energetic particles (SEP) and galactic cosmic rays (GCR) reach Mars’ surface, their total energy is ~104 times less than the solar UV energy. Exposure of organic compounds to direct UV radiation is one of the main degradation processes on Mars. The thin CO2 atmosphere allows UV with 190<λ<400 nm to reach the surface with a flux of ~1.4×1015 photons/s/cm2. Although the UV at the surface penetrates only millimeters into the regolith, its destructive power is more widely felt due to cryoturbation, wind, and impacts, allowing the regolith to be circulated and exposed to it.
The presence of organic compounds on Mars has now been reported by both Curiosity’s SAM/GCMS and Perseverance’s Scanning Habitable Environments with Raman & Luminescence for Organics & Chemicals (SHERLOC). The organic compounds include benzene, naphthalene, thiophenes, chlorinated aromatics, a variety of chloro-hydrocarbons [12-13A]. Most recently, preliminary results from the GCMS showed the possible presence of decane (C10H22), undecane (C11H24) and dodecane (C12H26), and suggested that a possible source may be from |
|
decomposition of long-chain carboxylic acids (e.g., 10-undecenoic acid) preserved in the mudstone [14]. It has been suggested that a possible sourcemay be the decomposition of long-chain carboxylic acids (e.g., 10-undecenoic acid). Even though their origin remains uncertain they could be derived from either abiotic or biotic sources.The identification of a large number of chlorinated compounds has been of special interest. There are currently three competing hypotheses as to the source of the detected chlorinated organic compounds [15]. These include: (1) terrestrial contamination that was chlorinated during the analysis; (2) martian organic matter that was chlorinated during analysis; and (3) martian organic compounds that were chlorinated prior to analysis by in-situ reactions with oxychlorines, their intermediary formation products, and/or radical species, produced during the formation or breakdown of oxychlorines.
The objectives of our research are to determine the extent of alteration or fragmentation of organic biomarker molecules under Mars ambient conditions:
(1) during the UV-driven production of oxychlorines from chloride-bearing salts; and
(2) in the presence of existing oxychlorines. We will also, for the first time, be using an actual martian sample in the form of sawdust from the EETA79001 Mars meteorite. The EETA79001 sawdust sample has already been received from the NASA-JSC meteorite curation center [6].
Although there are a number of studies on the effects of UV radiation on a variety of organic compounds identified as biomarkers [16-21], the effects of UV irradiation while oxychlorines are either being produced or present have not been previously studied. The results of our research will be of significance because we will for the first time be determining the extent of alteration or destruction of both biotic and abiotic organic compounds that could be present on Mars if life was present; biomarkers such as amino acids, simple and long-chain carboxylic acids (lipids), PAHs, hopanes, nucleobases, kerogen, and DNA/RNA. Over time, these biomarker molecules would have been exposed to diagenesis, radiation, or reactive oxidizing species, that would have altered or destroyed them. The original biomarker would have most likely been fragmented to new lower mass and simpler biomarkers. The question is: can we piece together these fragments from the original biomarker so that we can identify the parent biomarker with some level of confidence? Unfortunetly, the greater the fragmentation of the original biomarker, the more difficult it becomes to reconstruct it.
Our research, funded by NASA, will advance our understanding of the effects of oxychlorines and their UV-driven production on organic compounds and potential biomarkers on Mars, and thus advance our understanding of the extent of alteration or fragmentation of these compounds that should be present on Mars. A comprehensive understanding of the interactions between oxychlorine species and organics will impact our insight into the questions of what biomarkers we might expect to find in martian regolith and whether or not they would be chlorinated. Our results will help answer the questions about the presence of organic compounds and extant life on Mars, as well as providing a framework for interpreting the results of future landed Mars missions that search for biomarkers and life.

|
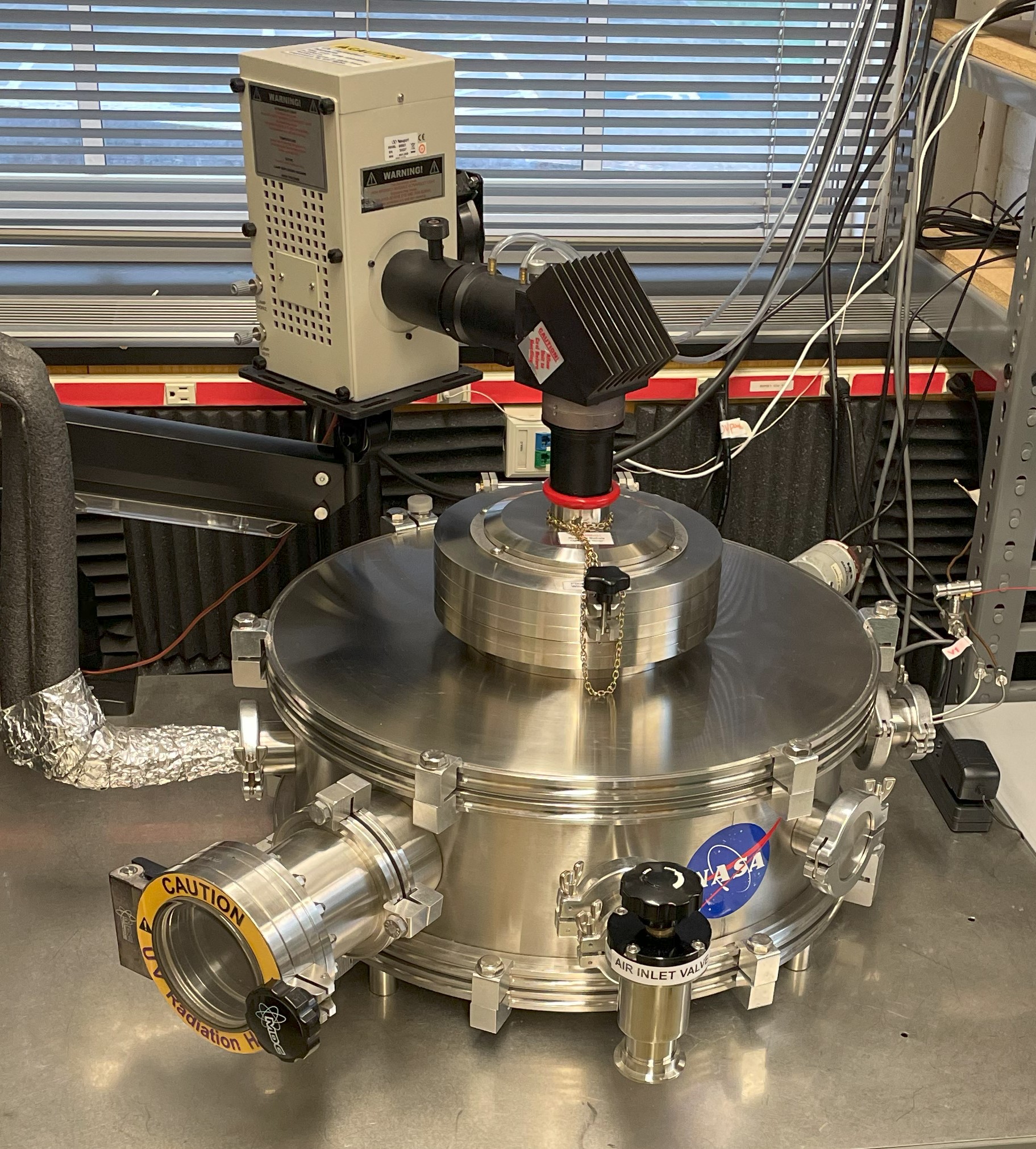
The Tufts Mars Simulation Chamber (MSC):
The MSC consists of a stainless steel cylindrical chamber with an internal depth of 45 cm and 60 cm diameter (~ 1.3×10-5 cm3). Samples are staged on a 25 cm diameter cold-plate whose temperature is controlled by an FTS RC210C0 recirculating cooler that can maintain temperatures as low as -80°C. A Mars gas mixture (CO2 95.3%, N2 2.8%, Ar 1.8% and O2 0.10%) can be introduced into the chamber at a constant flow rate. Pressure can be maintained between 1000-0.1 mbar monitored by an MKS baratron and controller connected to a scroll pump. Relative humidity (RH) and temperature are monitored by a Lascar EasyLog USB data logger and RH by a Decagon RH Sensor.
A Mars solar intensity spectrum is produced by 400 or 1000W xenon-arc lamps and delivered, via an IR filter, beam turner, and cutoff filters, through a 22 cm fused silica port on top of the chamber. The level of UV radiation (190-400 nm) is monitored using a Stellarnet UV BLUE-Wave Fiber Optic Spectrometer in order to correlate exposure time in the MSC to equivalent Mars UV dosages. Chamber gas composition and gaseous products can be monitored in real-time using an SRS QMS200 Mass Spectrometer. |
|

|
[0] "A Proposed Geobiology-Driven Nomenclature for Astrobiological In-Situ Observations and Sample Analyses" S. M. Perl et al., Astrobiology, 2021, 21, 954-966, doi: 10.1089/ast.2020.2318. Full Text
[1] "The Search for Organic Substances and Inorganic Volatile Compounds in the Surface of Mars" K. Biemann, et al., J. Geophys. Res., 1977, 82(28), 4641-4658, doi:10.1029/JS082i028p04641. Full Text
[2] "Combustion of Organic Molecules by the Thermal Decomposition of Perchlorate Salts: Implications for Organics at the Mars Phoenix
Scout Landing Site" D. W. Ming, et al., 2009, Abstract 2241, 40th Lunar Planet. Sci. Conf., Houston, TX. Full Text
[3] "Wet Chemistry Experiments on the 2007 Phoenix Mars Scout Lander: Data Analysis and Results" S. P. Kounaves, et al., J. Geophys. Res., 2010, 115, E00E10, doi:10.1029/2009JE003424. Full Text
[4] "Detection of Perchlorate and the Soluble Chemistry of Martian Soil at the Phoenix Lander Site" M. H. Hecht, S. P. Kounaves, et al., Science, 2009, 325, 64-67. Full Text PDF
[5] "Identification of the Perchlorate Parent Salts at the Phoenix Mars Landing Site and Possible Implications", S. P. Kounaves, et al., Icarus, 2014, 232, 226-231, doi:10.1016/j.icarus.2014.01.016. Full Text
[6] "Evidence of Martian Perchlorate, Chlorate, and Nitrate in Mars Meteorite EETA79001" S. P. Kounaves, B. L. Carrier, G. D. O’Neil,
S. T. Stroble, and M. W. Claire, Icarus, 2014, 229, 206-213, doi:10.1016/j.icarus.2013.11.012. Full Text
[7] "Indigenous Organic-Oxidized Fluid Interactions in the Tissint Mars Meteorite" E. A. Jaramillo, S. H. Royle, M. W. Claire, S. P. Kounaves,& M. A. Sephton, Geophys. Res. Lett., 2019, 46(6), 3090-3098, doi:10.1029/2018gl081335. Full Text
[8] "Evidence for Perchlorates and the Origin of Chlorinated Hydrocarbons Detected by SAM at the Rocknest Aeolian Deposit in Gale Crater" D. P. Glavin, et al., J. Geophys. Res., 2013, 118(10), 1955-1973, doi:10.1002/jgre.20144. Full Text
[9] "The Origins of Perchlorate in the Martian Soil" B. L. Carrier and S. P. Kounaves, Geophys. Res. Lett., 2015, 42, 3746-3754, doi:10.1002/2015GL064290. Full Text
[9A] "Permeation of photochemically-generated gaseous chlorine dioxide on Mars as a significant factor in destroying subsurface organic compounds", J. Newmark, and S. P. Kounaves, Nature Sci. Rep., 2024, 14(7), doi:10.1038/s41598-024-57968-1 Full Text
[10] "The Production of Perchlorate from Chlorite and Chlorate on Earth and Mars", D. Liu and S. P. Kounaves, ACS Earth Space Chem. 2019, 3, 1678-1684, doi:10.1021/acsearthspacechem.9b00134. Full Text
[10A] "Fragmentation of Tryptophan as a Biomarker on Mars when Exposed to UV in the Presence of an Oxychlorine", A. Walter and S. P. Kounaves, 54th Lunar & Planetary Science Conference, March 13–17, 2023, Abstract 2634. Full Text
[11] "Degradation of Amino Acids on Mars by UV Irradiation in the Presence of Chloride and Oxychlorine Salts", D. Liu and S. P. Kounaves, Astrobiology. 2021, 21, 793-801. doi:10.1089/ast.2020.2328. Full Text
[12] "Organic Molecules in the Sheepbed Mudstone, Gale Crater, Mars" C. Freissinet, et al., J. Geophys. Res., 2015, 120(3), 495-514, doi:10.1002/2014JE004737. Full Text
[13] "Organic Matter Preserved in 3-billion-year-old Mudstones at Gale Crater, Mars" J. L. Eigenbrode, et al., Science, 2018, 360, 1096-1101, doi:10.1126/science.aas9185. Full Text
[13A] "Aqueous alteration processes in Jezero crater, Mars: implications for organic geochemistry, E. L. Scheller, et al. Science, 2022, 378(6624), 1105-1110, doi:10.1126/science.abo5204. Full Text
[14] "Detection of Long-Chain Hydrocarbons on Mars with the Sample Analysis at Mars (SAM) Instrument" C. Freissinet, et al., Ninth International Conference on Mars, 2019, LPI, Pasadena, CA. Full Text PDF
[15] "Survivability of 1-Chloronapthalene During Simulated Early Diagenesis: Implications for Chlorinated Hydrocarbon Detection on Mars", S. H Royle, J. Tan, S. P. Kounaves, M. A. Sephton, J. Geophys. Res., 2018, 123, 2790-2802, doi:10.1029/2018JE005711. Full Text
[16] "UV irradiation of biomarkers adsorbed on minerals under Martian-like conditions: Hints for life detection on Mars" T. Fornaro, et al.,Icarus, 2018, 313, 38-60, doi:10.1016/j.icarus.2018.05.001. Full Text
[17] "Laboratory insights into the chemical and kinetic evolution of several organic molecules under simulated Mars surface UV radiation conditions" O. Poch, et al., Icarus, 2014, 242(0), 50-63, doi:10.1016/j.icarus.2014.07.014. Full Text
[18] "Chemical evolution of organic molecules under Mars-like UV radiation conditions simulated in the laboratory" O. Poch, et al., Planet. Space Sci., 2013, 85, 188-197, doi:10.1016/j.pss.2013.06.013. Full Text
[19] "Investigating the Photostability of Carboxylic Acids Exposed to Mars Surface Ultraviolet Radiation Conditions" F. Stalport, et al., Astrobiology, 2009, 9(6), 543-549, doi: 10.1089/ast.2008.0300. Full Text
[20] "The Martian Near Surface Environment: Analysis of Antarctic Soils and Laboratory Experiments on Putative Martian Organics" P. D. Archer, PhD Dissertation, University of Arizona, 2010. Full Text
[21] "UV Photolysis of Mellitic Acid - A Possible Organic at the mars Phoenix Landing Site" P. D. Archer et al., 40th Lunar and Planetary Science Conference, Abstract No. 2077, 2009. Full Text
|
|